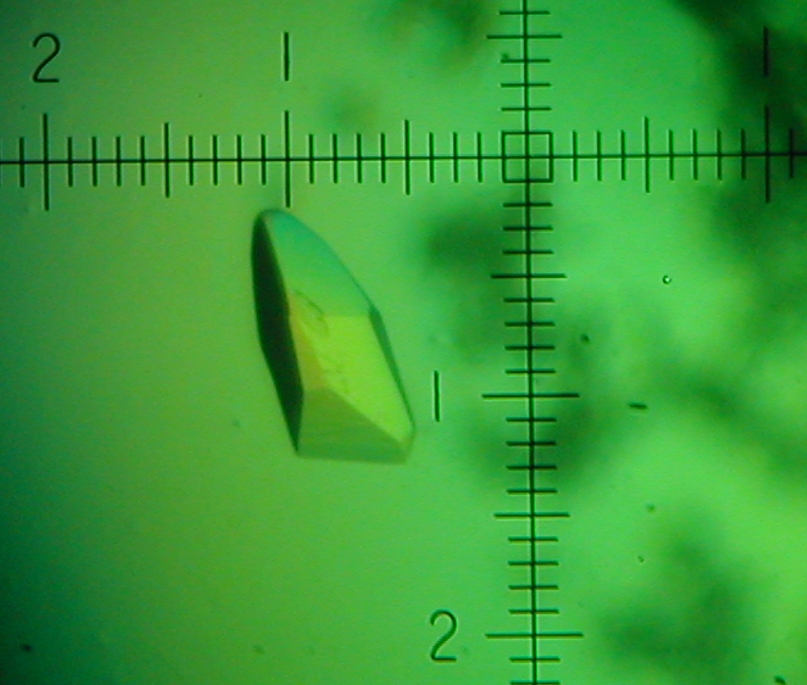
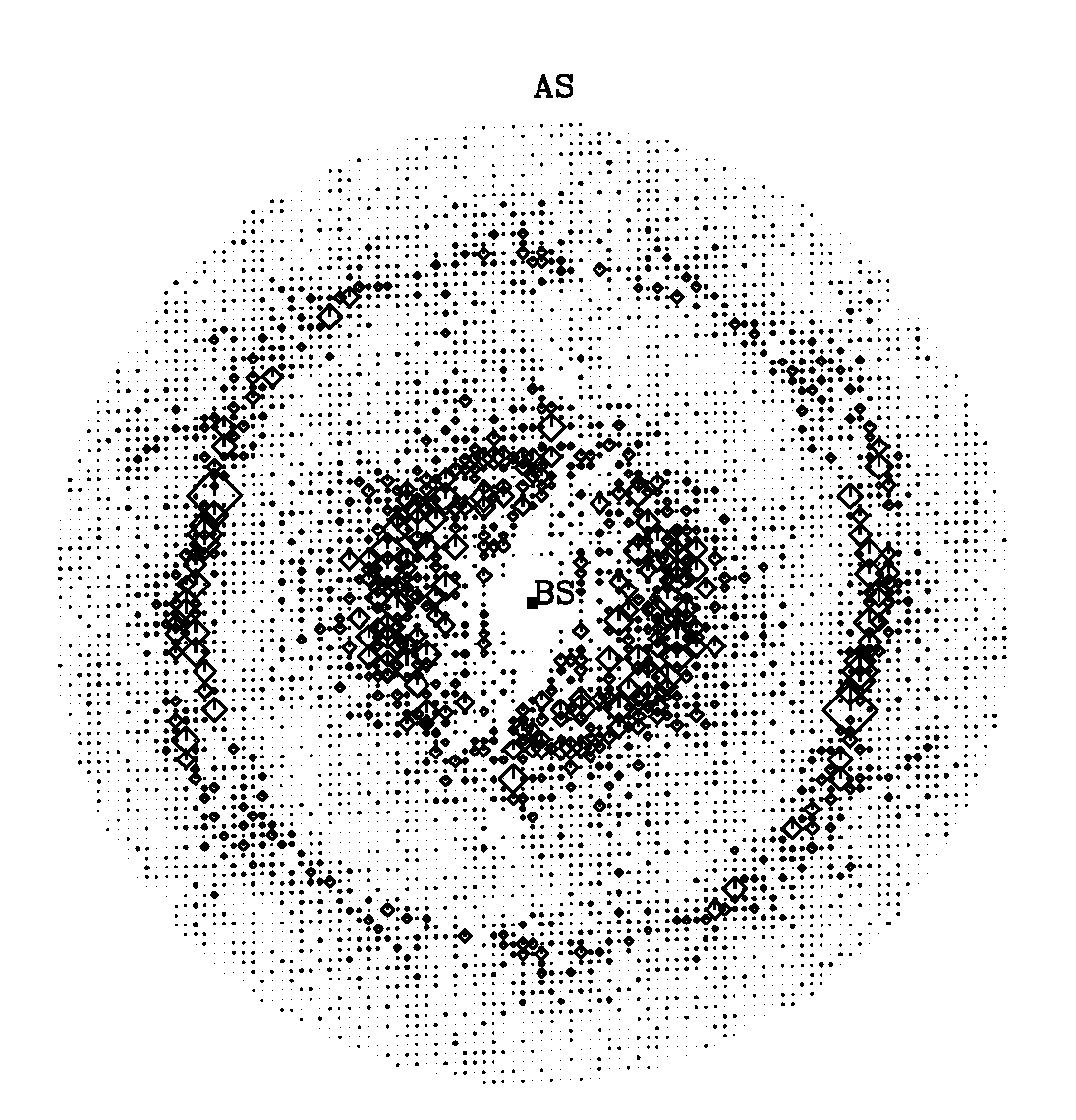
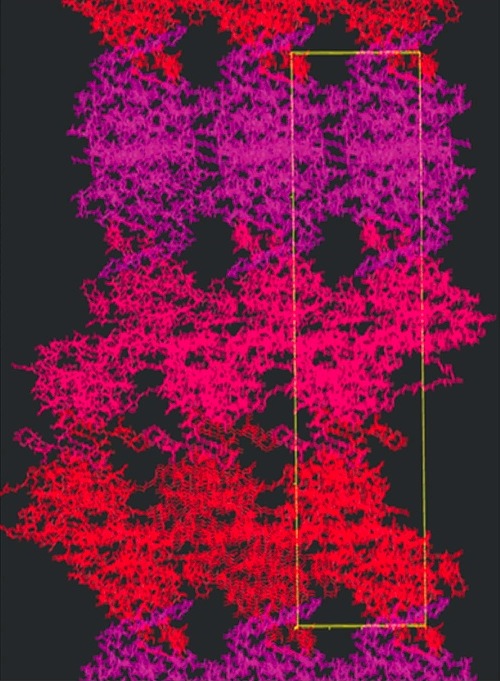
Welcome to the Gill-Lab website at The George Washington University and The George Washington Medical Faculty Associates. We integrate basic research with clinical and translational research by fostering an interdisplinary approach expertise that covers a wide range of levels of reductionism from X-ray crystallography to Molecular Biology to Confocal Imaging to Human Pathology and Treatment. The tabs to the right are links to discussions on various diseases ranging from Acidemia to Eye Disorders to Tuberculosis. Our main focus on this first tab is on how integral-membrane proteins in the kidney and eye function at the atomic level and to use this knowledge to implement diagnostics and therapuetics for human diseases and conditions such as type-II RTA and a myriad of eye disorders (glaucoma, band kerathapy, cataracts).
|
What's trending?
|
 Harry Gill, Ph.D.
Harry Gill, Ph.D.